Solid-state NMR of membrane peptides and proteins in the lipid cubic phase.
Ramberg, K.O., Boland, C., Kooshapur, H., Soubias, O., Wiktor, M., Huang, C.Y., Bailey, J., Gawrisch, K., Caffrey, M.(2025) Biophys J 124: 1387-1400
- PubMed: 40119522
- DOI: https://doi.org/10.1016/j.bpj.2025.03.012
- Primary Citation of Related Structures:
9EMZ - PubMed Abstract:
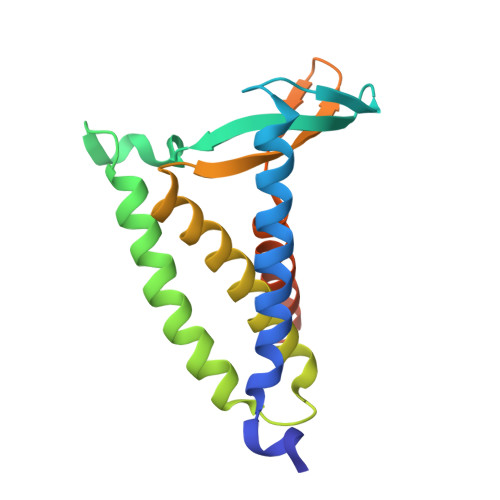
Solid-state nuclear magnetic resonance (ssNMR) is a powerful technique for studying membrane protein structure and dynamics. Ideally, measurements are performed with the protein in a lipid bilayer. However, homogenous reconstitution of functional protein into intact bilayers at sufficiently high concentrations is often difficult to achieve. In this work, we investigate the suitability of the lipid cubic phase (LCP), which incorporates a lipid bilayer, as an alternative medium for ssNMR of integral membrane peptides and proteins. The cubic mesophase has long been used to generate membrane protein crystals for use in X-ray crystallographic structure determination by the so-called in meso method, and for protein functional and biophysical characterization. Preparing and handling protein-laden LCP is straightforward. LCP may therefore provide a valuable alternative to native membranes and other membrane mimetics for ssNMR. We tested this idea by conducting standard magic angle spinning (MAS) ssNMR experiments on LCP into which gramicidin, a ∼4 kDa transmembrane peptide, or bacterial lipoprotein signal peptidase II (LspA), a ∼20 kDa integral membrane enzyme, had been reconstituted. We report one- and two-dimensional ssNMR spectra for both gramicidin and LspA and the parameters for optimizing spectral quality. The high protein carrying capacity of the cubic phase facilitated 13 C ssNMR at natural abundance. Lowering temperature and raising MAS frequency enabled significant improvements in spectral quality. One-dimensional 13 C and 15 N spectra were collected for LspA. Two-dimensional ssNMR experiments provided information on LspA dynamics and its interaction with the water and lipid components of the cubic phase. Solution NMR measurements carried out in parallel yielded information on the effect of the antibiotic, globomycin, on LspA structure and dynamics.
- Membrane Structural & Functional Biology Group, School of Medicine and School of Biochemistry & Immunology, Trinity College Dublin, Dublin, D02 R590, Ireland. Electronic address: martin.caffrey@tcd.ie.
Organizational Affiliation: