Novel Humanized Anti-HER3 Antibodies: Structural Characterization and Therapeutic Activity.
Muzi, A., Arriga, R., Bulfaro, G., Fata, F., Costanzo, A., Chiarini, V., Cappelletti, M., Ferrara, F.F., Bucci, F., Montemiglio, L.C., Savino, C., Marra, E., Ciliberto, G., Aurisicchio, L., Vallone, B., Roscilli, G.(2025) Antibodies (Basel) 14
- PubMed: 41133678
- DOI: https://doi.org/10.3390/antib14040084
- Primary Citation of Related Structures:
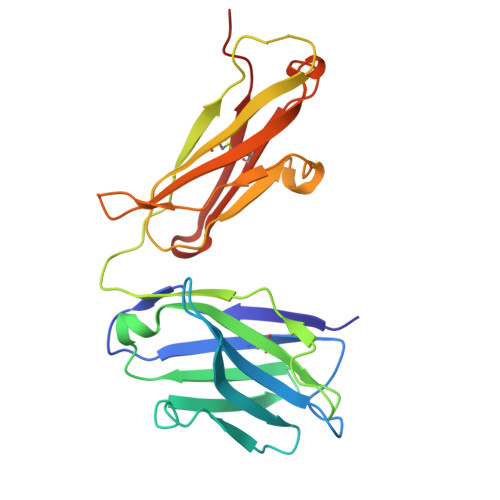
9I1Q - PubMed Abstract:
The ErbB protein family plays a critical role in the progression of various solid tumors, and HER3 has been implicated in resistance mechanisms to multiple cancer therapies due to its ability to form heterodimers with other ErbB receptors, thereby activating pathways that promote tumor growth and survival. This study aimed to generate and characterize humanized monoclonal antibodies against HER3 to inhibit its function and evaluate their potential as therapeutic agents. Murine monoclonal antibodies TK-A3 and TK-A4 were humanized and tested for binding to ErbB3 and competition with neuregulin-1β (NRG). Specificity was assessed by ELISA, and epitope identified by X-ray crystallography. Downstream signaling was analyzed by western blot for phosphorylated ErbB3, Akt, and MAPK. Antitumor activity was evaluated in vitro and in a pancreatic cancer xenograft model. A toxicology study was also conducted. TK-hu A3 and TK-hu A4 bound specifically to ErbB3 without cross-reactivity to other ErbB receptors. The ErbB3-TK-hu A3 Fab structure revealed the binding epitope. Both antibodies competed with NRG, inhibiting ErbB3, Akt, and MAPK phosphorylation in a dose-dependent manner. They suppressed cancer cell survival in vitro, and TK-hu A3 significantly delayed tumor growth in vivo. The toxicology study indicated good tolerability. TK-hu A3 emerged as the lead candidate, showing specific HER3 targeting, strong pathway inhibition, and antitumor efficacy in vivo. Beyond standalone use, it could support novel strategies such as T-cell engagers, ADCs, CAR-T, and bispecific antibodies. These findings highlight TK-hu A3 as a promising therapy for HER3-positive, treatment-resistant cancers, meriting further development.
- Takis s.r.l., 00128 Rome, Italy.
Organizational Affiliation: