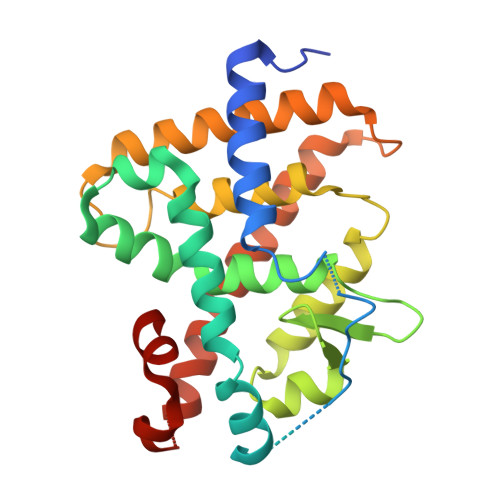
Discovery of a Potent, Selective, and Multiple His435 Mutation-Sensitive Thyroid Hormone Receptor beta Agonist.
Li, Q., Yao, B., Zhao, S., Lu, Z., Wu, X., Lu, Z., Wu, T., Li, J., Chen, X., Chen, Z., Zhang, C., Wu, D., Zhang, Y., Xiang, Q., Li, Y., Xu, Y.(2025) J Med Chem
- PubMed: 40539969
- DOI: https://doi.org/10.1021/acs.jmedchem.5c00164
- Primary Citation of Related Structures:
9IX5, 9J0K - PubMed Abstract:
Beyond selectivity concerns for thyroid hormone receptor β (THR-β) agonists, intolerance to the His435 mutation remains a challenge. Following our previous study, we performed detailed modifications on the 7-position of isoquinoline, specifically targeting the hydrophobic region of the THR-β ligand-binding pocket (LBP). This led to the identification of compound 15n , which showed potent THR-β agonistic activity (EC 50 : 3.2 nM), moderately selectivity (∼10-fold), and good activation of multiple His435 mutants (EC 50 : 134.2 nM to 515.5 nM). Co-crystal structures revealed that the introduction of small-volume groups into the hydrophobic pocket of THR-β almost did not significantly displace helix 11 or helix 3, explaining why 15n can activate multiple His435 mutants simultaneously. Multiple experiments confirmed that 15n exhibits excellent lipid metabolism, safety, and pharmacokinetic properties. Together, 15n emerges as a potent, selective, and His435 mutation-sensitive THR-β agonist, offering potential for treating dyslipidemia, metabolic dysfunction-associated steatohepatitis (MASH), or resistance to thyroid hormone (RTH).
Organizational Affiliation:
China-New Zealand Joint Laboratory of Biomedicine and Health, State Key Laboratory of Respiratory Disease, Guangdong Provincial Key Laboratory of Biocomputing, Institute of Drug Discovery, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, No. 190 Kaiyuan Avenue, Guangzhou 510530, China.