Structural and Functional Insights into Targeting GCCG Sites in the EGFR Promoter by Two DNA Intercalators to Inhibit Breast Cancer Metastasis.
Chang, C.C., Li, H.J., Satange, R., Lin, S.M., Chen, T.L., Lin, C.C., Neidle, S., Hou, M.H.(2025) J Med Chem 68: 6601-6615
- PubMed: 40032551
- DOI: https://doi.org/10.1021/acs.jmedchem.4c03203
- Primary Citation of Related Structures:
9JL7 - PubMed Abstract:
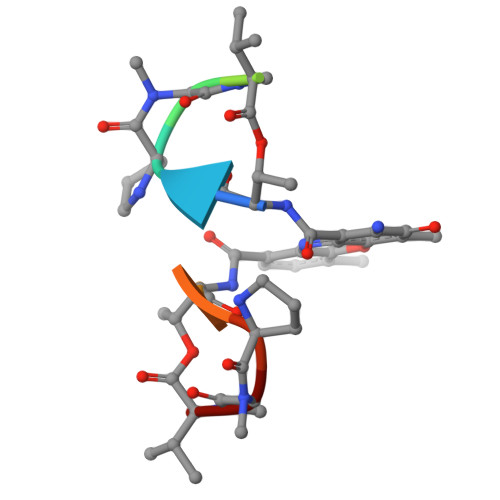
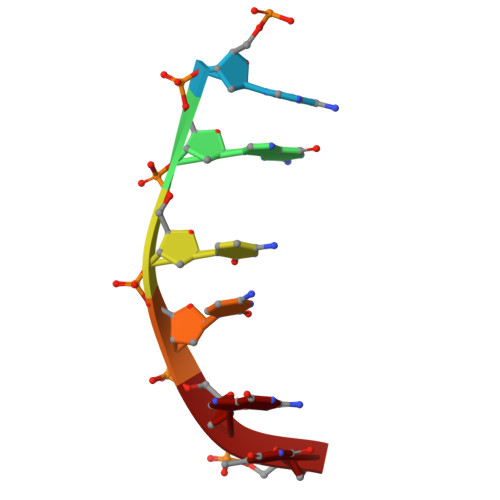
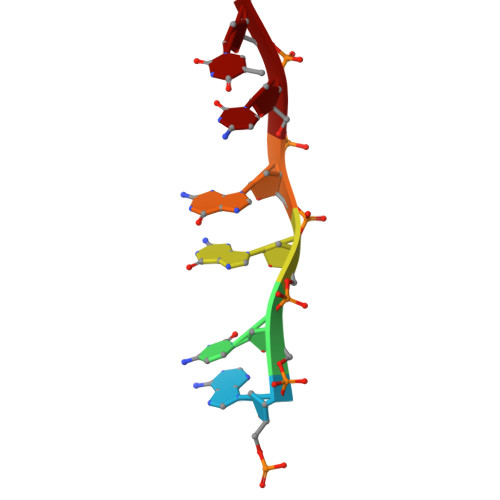
Chemotherapeutic drugs are commonly used to treat cancers lacking targeted therapy options. However, their low specificity limits their treatment effectiveness. We report here that the cooperative binding of doxorubicin (Dox) with actinomycin D (ActD) enhances the specificity for consecutive GCCG sites in DNA. Using X-ray crystallography, we determined the crystal structure of ActD and Dox bound to d(AGCCGT) 2 DNA. ActD intercalation at the GpC site induces a novel Dox binding mode at the adjacent CpG step. This ensures a snug fit, avoids steric clashes, and enhances the specificity. Transcriptome analysis revealed that combining Dox with ActD synergistically down-regulates EGFR in TNBC cells. Additionally, it reduces EGFR promoter activity. In vivo, the combination significantly suppresses tumor growth and outperforms the standard Dox and cyclophosphamide regimen in inhibiting metastasis. This study highlights targeting the activated EGFR pathway with sequence-specific DNA-targeting drug combinations as a potential TNBC treatment.
- Graduate Institute of Biotechnology, National Chung Hsing University, Taichung 402, Taiwan.
Organizational Affiliation: