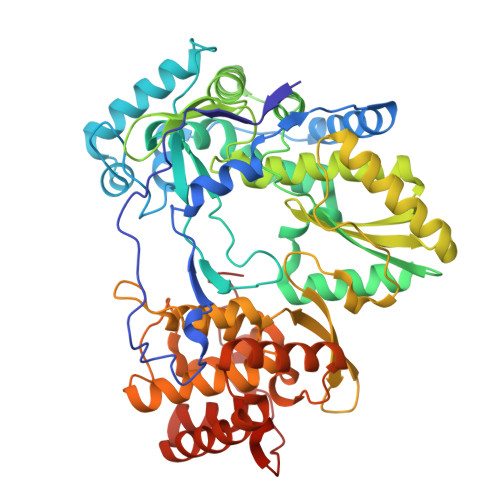
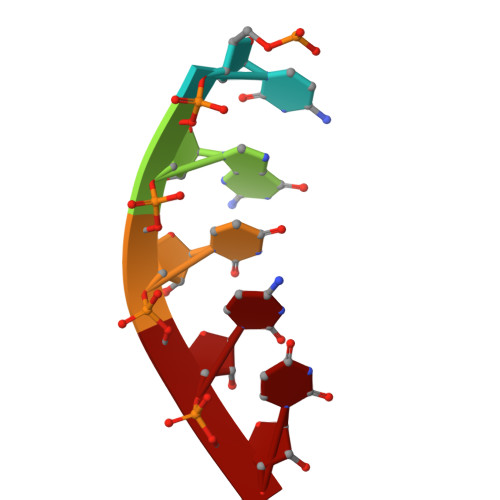
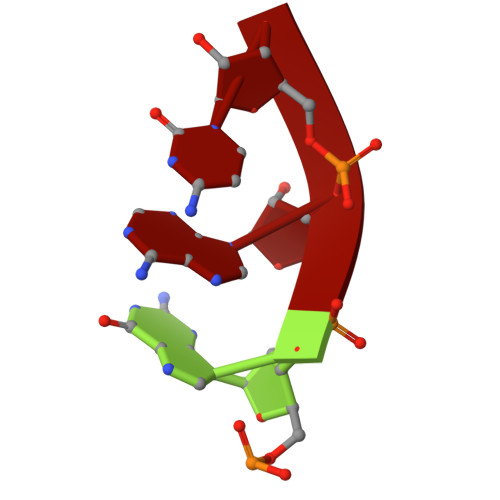
Structural insights into polymerase-catalyzed FAD capping of hepatitis C virus RNA.
Wang, D.P., Zhao, R., Hu, W.S., Li, H.N., Cao, J.M., Zhou, X., Xiang, Y.(2025) Nat Commun 16: 7298-7298
- PubMed: 40775214
- DOI: https://doi.org/10.1038/s41467-025-62609-w
- Primary Citation of Related Structures:
9LJR, 9LJS, 9LJT, 9LJU, 9LJV, 9LJW - PubMed Abstract:
The RNA polymerase NS5B of HCV is capable of catalyzing the addition of flavin adenine dinucleotide (FAD) to its RNA as a 5' cap structure, aiding the virus in evading host immune responses. However, the exact mechanism underlying the 5'-FAD capping process of HCV RNA remains to be elucidated. Here, we determine crystal structures of the HCV NS5B de novo initiation, primed initiation and elongation complexes in presence of FAD. Structural analysis and comparisons show that residues M447 and Y448 of the β loop in the priming element (PE) of NS5B are the determinants for specific recognition of FAD. The adenine group of FAD is exclusively paired with the uracil base at the 3' end of the template RNA strand. At the initial elongation stage, the C-terminal linker (residues 530-570) of NS5B is involved in stabilizing the 5' FAD, which in turn induces sequential conformational changes of the bases in the product strand and creates a unique intermediate state of the RNA duplex, facilitating the translocation of the product strand. Our study offers novel insights for developments of new anti-HCV therapies.
- Department of Cardiology, The First Hospital of Shanxi Medical University, MOE Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, China.
Organizational Affiliation: