Cryo-EM visualization of RAD51 filament assembly and end-capping by XRCC3-RAD51C-RAD51D-XRCC2
Greenhough, L.A., Galanti, L., Liang, C.C., Boulton, S.J., West, S.C.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA repair protein RAD51 homolog 4 | A [auth B] | 328 | Homo sapiens | Mutation(s): 0 Gene Names: RAD51D, RAD51L3 | 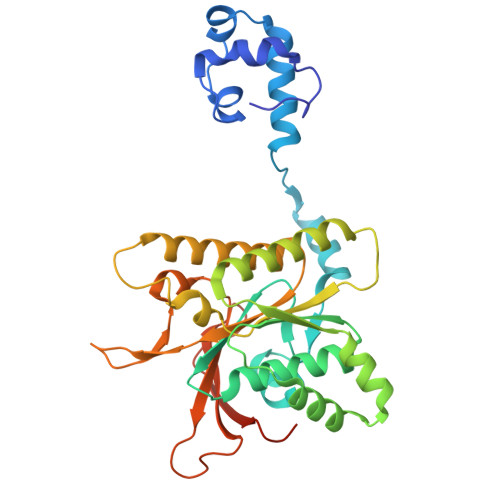 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O75771 (Homo sapiens) Explore O75771 Go to UniProtKB: O75771 | |||||
PHAROS: O75771 GTEx: ENSG00000185379 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O75771 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA repair protein RAD51 homolog 3 | D [auth C] | 376 | Homo sapiens | Mutation(s): 0 Gene Names: RAD51C, RAD51L2 |  |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O43502 (Homo sapiens) Explore O43502 Go to UniProtKB: O43502 | |||||
PHAROS: O43502 GTEx: ENSG00000108384 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O43502 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA repair protein XRCC3 | E [auth D] | 346 | Homo sapiens | Mutation(s): 0 Gene Names: XRCC3 | 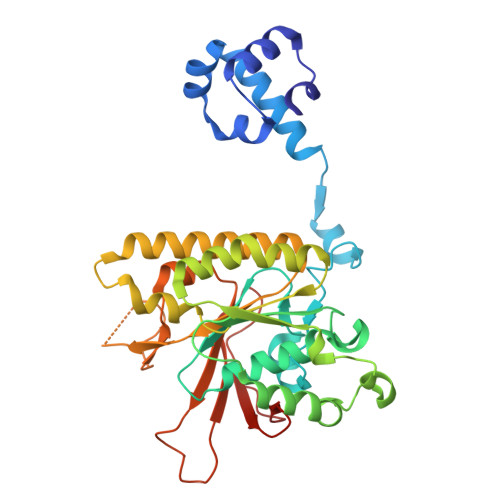 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O43542 (Homo sapiens) Explore O43542 Go to UniProtKB: O43542 | |||||
PHAROS: O43542 GTEx: ENSG00000126215 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O43542 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA repair protein XRCC2 | F [auth A] | 280 | Homo sapiens | Mutation(s): 0 Gene Names: XRCC2 | 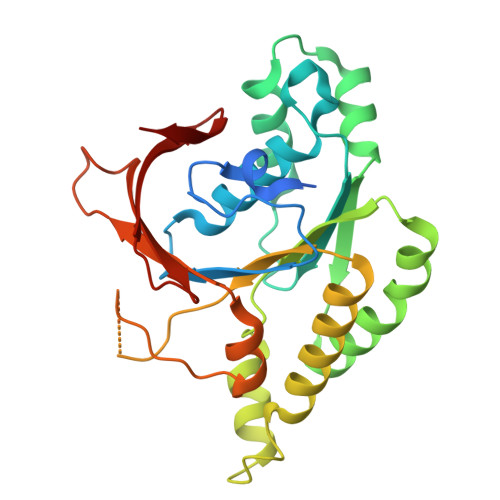 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O43543 (Homo sapiens) Explore O43543 Go to UniProtKB: O43543 | |||||
PHAROS: O43543 GTEx: ENSG00000196584 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O43543 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 7 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA repair protein RAD51 homolog 1 | 339 | Homo sapiens | Mutation(s): 0 Gene Names: RAD51, RAD51A, RECA |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q06609 (Homo sapiens) Explore Q06609 Go to UniProtKB: Q06609 | |||||
PHAROS: Q06609 GTEx: ENSG00000051180 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q06609 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| dN-31nt | B [auth M] | 31 | synthetic construct |  | |
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| dN-21nt | C [auth N] | 21 | synthetic construct |  | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ATP (Subject of Investigation/LOI) Query on ATP | BA [auth G] EA [auth H] HA [auth I] KA [auth J] N [auth B] | ADENOSINE-5'-TRIPHOSPHATE C10 H16 N5 O13 P3 ZKHQWZAMYRWXGA-KQYNXXCUSA-N |  | ||
| ADP (Subject of Investigation/LOI) Query on ADP | P [auth C] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| CA (Subject of Investigation/LOI) Query on CA | CA [auth G] FA [auth H] IA [auth I] LA [auth J] NA [auth K] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | AA [auth F] DA [auth G] GA [auth H] JA [auth I] MA [auth J] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.21_5207 |
| RECONSTRUCTION | cryoSPARC |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Wellcome Trust | United Kingdom | CC2098 |
| Medical Research Council (MRC, United Kingdom) | United Kingdom | CC2098 |
| Wellcome Trust | United Kingdom | CC2098 |
| Biotechnology and Biological Sciences Research Council (BBSRC) | United Kingdom | BB/W01355X/1 |