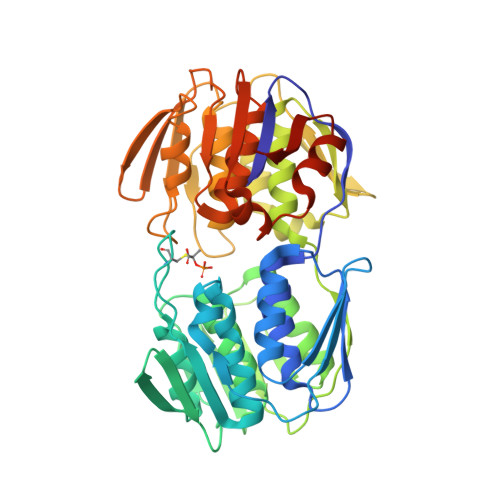
Enolpyruvate transferase MurAA A149E , identified during adaptation of Enterococcus faecium to daptomycin, increases stability of MurAA-MurG interaction.
Zhou, Y., Utama, B., Pratap, S., Supandy, A., Song, X., Tran, T.T., Mehta, H.H., Arias, C.A., Shamoo, Y.(2023) J Biological Chem 299: 102912-102912
- PubMed: 36649910
- DOI: https://doi.org/10.1016/j.jbc.2023.102912
- Primary Citation of Related Structures:
7TB0, 8D84 - PubMed Abstract:
Daptomycin (DAP) is an antibiotic frequently used as a drug of last resort against vancomycin-resistant enterococci. One of the major challenges when using DAP against vancomycin-resistant enterococci is the emergence of resistance, which is mediated by the cell-envelope stress system LiaFSR. Indeed, inhibition of LiaFSR signaling has been suggested as a strategy to "resensitize" enterococci to DAP. In the absence of LiaFSR, alternative pathways mediating DAP resistance have been identified, including adaptive mutations in the enolpyruvate transferase MurAA (MurAA A149E ), which catalyzes the first committed step in peptidoglycan biosynthesis; however, how these mutations confer resistance is unclear. Here, we investigated the biochemical basis for MurAA A149E -mediated adaptation to DAP to determine whether such an alternative pathway would undermine the potential efficacy of therapies that target the LiaFSR pathway. We found cells expressing MurAA A149E had increased susceptibility to glycoside hydrolases, consistent with decreased cell wall integrity. Furthermore, structure-function studies of MurAA and MurAA A149E using X-ray crystallography and biochemical analyses indicated only a modest decrease in MurAA A149E activity, but a 16-fold increase in affinity for MurG, which performs the last intracellular step of peptidoglycan synthesis. Exposure to DAP leads to mislocalization of cell division proteins including MurG. In Bacillus subtilis, MurAA and MurG colocalize at division septa and, thus, we propose MurAA A149E may contribute to DAP nonsusceptibility by increasing the stability of MurAA-MurG interactions to reduce DAP-induced mislocalization of these essential protein complexes.
- Department of Biosciences, Rice University, Houston, Texas, USA.
Organizational Affiliation: