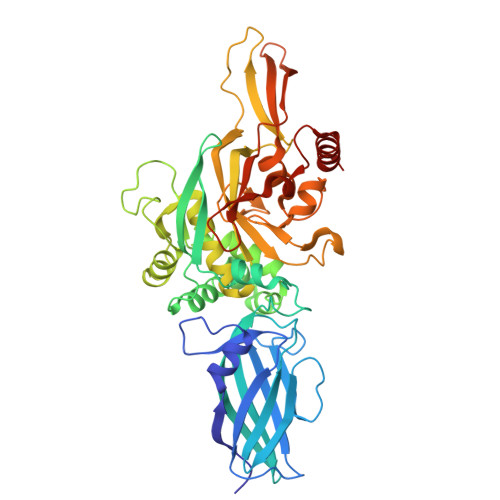
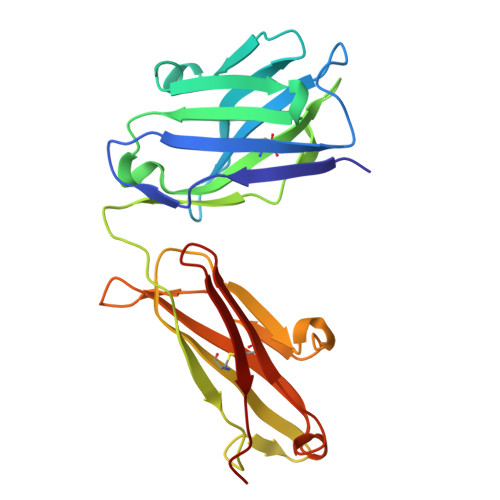
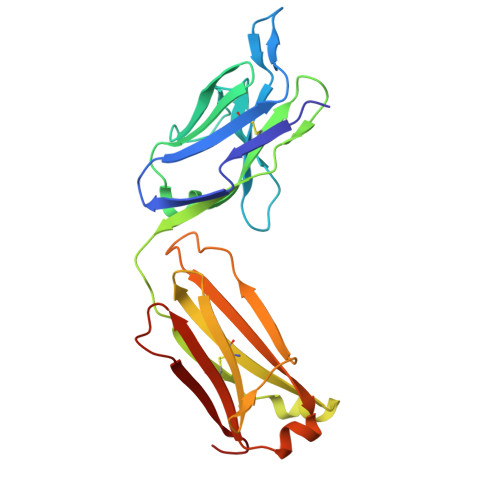
Enzyme-activating B-cell receptors boost antigen presentation to pathogenic T cells in gluten-sensitive autoimmunity.
Iversen, R., Heggelund, J.E., Das, S., Hoydahl, L.S., Sollid, L.M.(2025) Nat Commun 16: 2387-2387
- PubMed: 40064932
- DOI: https://doi.org/10.1038/s41467-025-57564-5
- Primary Citation of Related Structures:
8RMX, 8RMY - PubMed Abstract:
Autoantibodies against the enzyme transglutaminase 3 (TG3) are characteristic to the gluten-sensitive skin disorder dermatitis herpetiformis (DH), which is an extraintestinal manifestation of celiac disease. We here demonstrate that TG3-specific B cells can activate gluten-specific CD4 + T cells through B-cell receptor (BCR)-mediated internalization of TG3-gluten enzyme-substrate complexes. Stereotypic anti-TG3 antibodies using IGHV2-5/IGKV4-1 gene segments enhance the catalytic activity of TG3, and this effect translates into increased gluten presentation to T cells when such antibodies are expressed as BCRs. The crystal structure of TG3 bound to an IGHV2-5/IGKV4-1 Fab shows that antibody binding to a β-sheet in the catalytic core domain causes the enzyme to adopt the active conformation. This mechanism explains the production of stereotypic anti-TG3 autoantibodies in DH and highlights a role for TG3-specific B cells as antigen-presenting cells for gluten-specific T cells. Similar boosting effects of autoreactive BCRs could be relevant for other autoimmune diseases, including rheumatoid arthritis.
- Norwegian Coeliac Disease Research Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway. rasmus.iversen@medisin.uio.no.
Organizational Affiliation: