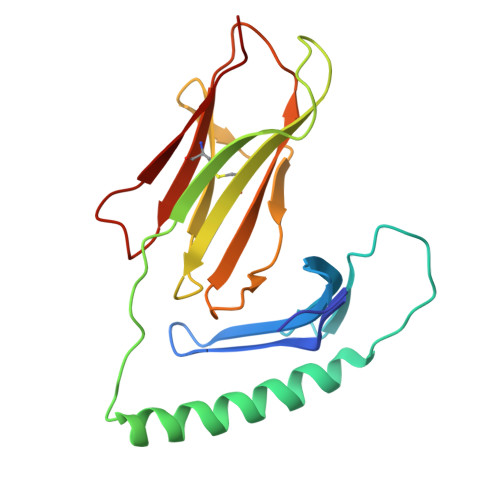
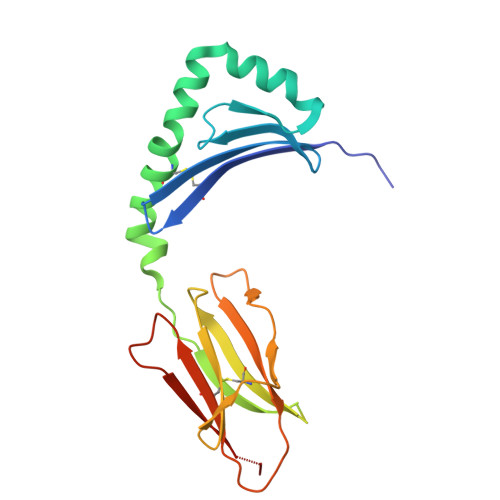
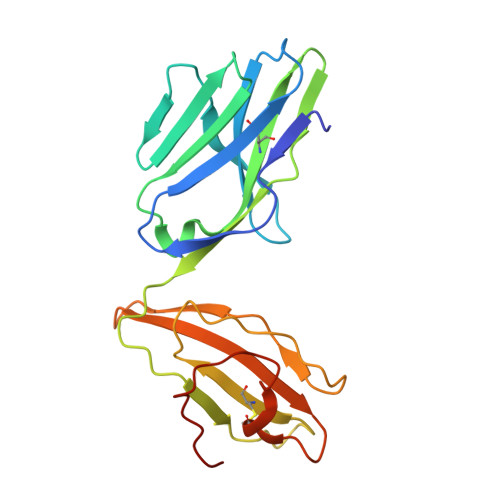
A naturally selected alpha beta T cell receptor binds HLA-DQ2 molecules without co-contacting the presented peptide.
Lim, J.J., Jones, C.M., Loh, T.J., Dao, H.T., Tran, M.T., Tye-Din, J.A., La Gruta, N.L., Rossjohn, J.(2025) Nat Commun 16: 3330-3330
- PubMed: 40199885
- DOI: https://doi.org/10.1038/s41467-025-58690-w
- Primary Citation of Related Structures:
9EJG, 9EJH, 9EJI - PubMed Abstract:
αβ T cell receptors (TCR) co-recognise peptide (p) antigens that are presented by major histocompatibility complex (MHC) molecules. While marked variations in TCR-p-MHC docking topologies have been observed from structural studies, the co-recognition paradigm has held fast. Using HLA-DQ2.5-peptide tetramers, here we identify a TRAV12-1 + -TRBV5-1 + G9 TCR from human peripheral blood that binds HLA-DQ2.5 in a peptide-agnostic manner. The crystal structures of TCR-HLA-DQ2.5-peptide complexes show that the G9 TCR binds HLA-DQ2.5 in a reversed docking topology without contacting the peptide, with the TCR contacting the β1 region of HLA-DQ2.5 and distal from the peptide antigen binding cleft. High-throughput screening of HLA class I and II molecules finds the G9 TCR to be pan-HLA-DQ2 reactive, with leucine-55 of HLA-DQ2.5 being a key determinant underpinning G9 TCR specificity excluding other HLA-II allomorphs. Consistent with the functional assays, the interactions of the G9 TCR and HLA-DQ2.5 precludes CD4 binding, thereby impeding T cell activation. Collectively, we describe a naturally selected αβTCR from human peripheral blood that deviates from the TCR-p-MHC co-recognition paradigm.
- Infection and Immunity Program and Department of Biochemistry and Molecular Biology, Biomedicine Discovery Institute, Monash University, Clayton, VIC, Australia.
Organizational Affiliation: