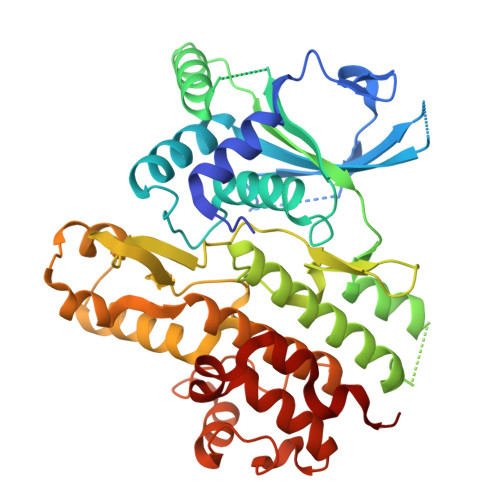
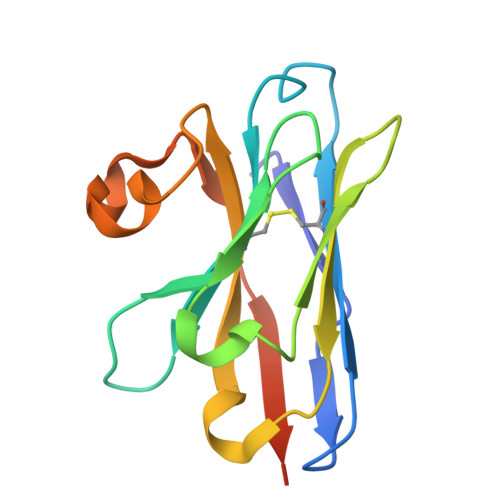
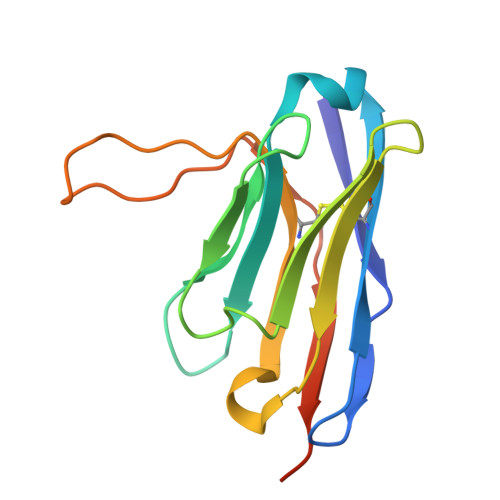
The fast-evolving FIKK kinase family of Plasmodium falciparum can be inhibited by a single compound.
Belda, H., Bradley, D., Christodoulou, E., Nofal, S.D., Broncel, M., Jones, D., Davies, H., Bertran, M.T., Purkiss, A.G., Ogrodowicz, R.W., Joshi, D., O'Reilly, N., Walport, L., Powell, A., House, D., Kjaer, S., Claessens, A., Landry, C.R., Treeck, M.(2025) Nat Microbiol 10: 1463-1483
- PubMed: 40389650
- DOI: https://doi.org/10.1038/s41564-025-02017-4
- Primary Citation of Related Structures:
9EMY - PubMed Abstract:
Of 250 Plasmodium species, 6 infect humans, with P. falciparum causing over 95% of 600,000 annual malaria-related deaths. Its pathology arises from host cell remodelling driven by over 400 exported parasite proteins, including the FIKK kinase family. About one million years ago, a bird-infecting Plasmodium species crossed into great apes and a single non-exported FIKK kinase gained an export element. This led to a rapid expansion into 15-21 atypical, exported Ser/Thr effector kinases. Here, using genomic and proteomic analyses, we demonstrate FIKK differentiation via changes in subcellular localization, expression timing and substrate motifs, which supports an individual important role in host-pathogen interactions. Structural data and AlphaFold2 predictions reveal fast-evolving loops in the kinase domain that probably enabled rapid functional diversification for substrate preferences. One FIKK evolved exclusive tyrosine phosphorylation, previously thought absent in Plasmodium. Despite divergence of substrate preferences, the atypical ATP binding pocket is conserved and we identified a single compound that inhibits all FIKKs. A pan-specific inhibitor could reduce resistance development and improve malaria control strategies.
- Signalling in Apicomplexan Parasites Laboratory, The Francis Crick Institute, London, UK.
Organizational Affiliation: