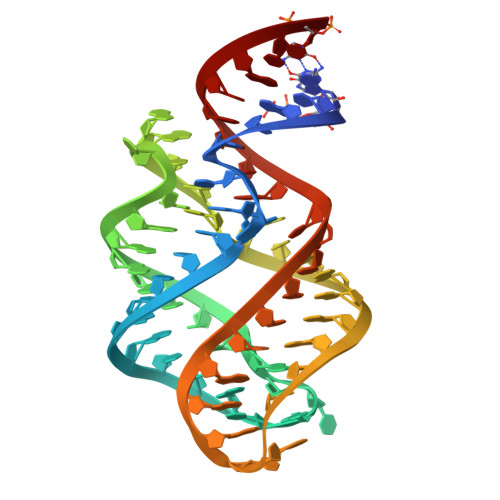
Structure-based insights into the ligand specificity tuning of 2'-dG-III riboswitch.
Shen, X., Li, H., Xu, X., Song, Q., Tai, X., He, M., Ren, A.(2025) Nucleic Acids Res 53
- PubMed: 40795954
- DOI: https://doi.org/10.1093/nar/gkaf773
- Primary Citation of Related Structures:
9LKU, 9LKV, 9LKW, 9UW0 - PubMed Abstract:
Riboswitches are conserved non-coding RNA domains predominantly located at the 5'-end of the bacterial mRNAs, serving as gene expression regulators. Recently, a third class of 2'-deoxyguanosine riboswitch (2'-dG-III) has been identified from guanine riboswitch family, exhibiting comparable binding affinity toward 2'-dG, guanine, and guanosine. To elucidate the unique ligand recognition mechanism of this riboswitch, we solved its crystal structures in complex with different purine derivatives, including 2'-dG, guanine, and guanosine. The tertiary structure reveals a typical tuning-fork-like architecture, with three stems converging at a central three-way junction. The bound ligand, 2'-dG, is anchored within the junctional core through specific molecular interactions involving certain critical nucleotides. Through systemic comparative analysis of the binding pocket across different ligand-bound states, as well as related guanine family riboswitches, including 2'-dG-I, 2'-dG-II, and Guanine-I riboswitches, we identified subtle yet significant structural variations that modulate binding affinity and specificity. Leveraging these findings, we further engineered RNA biosensors by fusing the 2'-dG-III riboswitch with Pepper fluorogenic RNA aptamer, which exhibits a robust, positive correlation between fluorescence intensity and 2'-dG levels in vitro. Together, this work not only advances our understanding of the ligand recognition mechanisms underlying the 2'-dG-III riboswitch and related guanine riboswitch family but also lays the groundwork for fine-tuning riboswitch specificity, paving the way for the development of highly specific RNA-based biosensors.
- Department of Cardiology of The Second Affiliated Hospital and Life Sciences Institute and School of Medicine, Zhejiang University, Hangzhou 310058, China.
Organizational Affiliation: