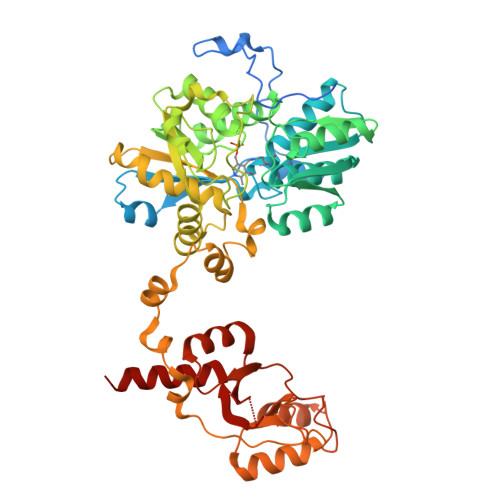
The disease-linked R336C mutation in cystathionine beta-synthase disrupts communication with the PLP cofactor, yet maintains the enzyme's overall structural integrity.
Conter, C., Nunez-Franco, R., Al-Sadeq, D.W., Fernandez-Rodriguez, C., Goikoetxea-Usandizaga, N., Nasrallah, G.K., Nomikos, M., Martinez-Chantar, M.L., Astegno, A., Jimenez-Oses, G., Martinez-Cruz, L.A.(2025) FEBS J 292: 4933-4954
- PubMed: 40327797
- DOI: https://doi.org/10.1111/febs.70116
- Primary Citation of Related Structures:
9HIF - PubMed Abstract:
Cystathionine β-synthase (CBS) is a pyridoxal-phosphate (PLP)-dependent enzyme essential for the reverse transsulfuration pathway, where homocysteine and serine combine to form cystathionine, the immediate precursor of cysteine. Mutations in the CBS gene cause homocystinuria, a disorder associated with intellectual disability, multisystem complications, and reduced life expectancy. The CBS p.R336C mutation, linked to severe pyridoxine non-responsiveness, results in reduced enzyme activity, previously attributed to protein instability and weakened substrate and PLP binding. To clarify the effects of the pathological R336C mutation, we performed biochemical, biophysical, and crystallographic analyses, as well as molecular dynamics simulations. Our findings show that the R336C mutation minimally impacts the structural environment around residue 336, does not cause enzyme misfolding, and does not impair the binding of PLP or the allosteric activator S-adenosylmethionine (AdoMet) binding. Instead, the mutation induces subtle reorientations in nearby hydrophobic residues, including F185 and Y381, altering intramolecular contacts that perturb the interaction between asparagine 149 and the O3 oxygen of PLP. This alteration is known to potentially shift the tautomeric equilibrium of the PLP Schiff base from its catalytically active ketoenamine form to the inactive enolimine form, which aligns with the reduced activity of the R336C variant. Additionally, the R336C mutation disrupts intermolecular contacts between the catalytic core and Bateman module, altering the Bateman module's intrinsic mobility in the enzyme's basal state and potentially affecting the cavity opening required for catalysis. Importantly, the R336C variant retains the native enzyme's ability to assemble into polymeric chains in crystals, preserving its filament formation capacity.
- Center for Cooperative Research in Biosciences (CIC bioGUNE), Basque Research and Technology Alliance (BRTA), Derio, Spain.
Organizational Affiliation: