High-resolution structure of human SHMT2 with covalently bound PLP (internal aldimine)
Warlich, A., Ruszkowski, M., Nawrot, D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Serine hydroxymethyltransferase, mitochondrial | 476 | Homo sapiens | Mutation(s): 0 Gene Names: SHMT2 EC: 2.1.2.1 | 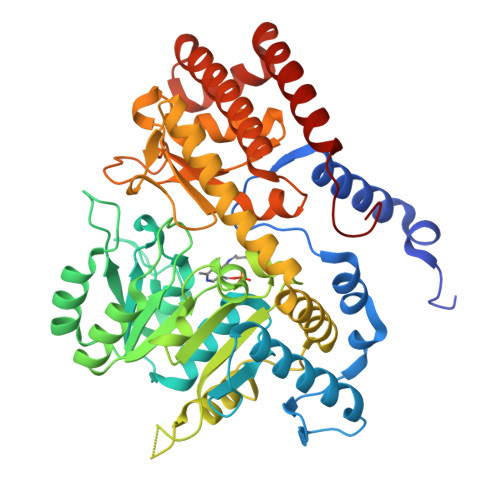 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P34897 (Homo sapiens) Explore P34897 Go to UniProtKB: P34897 | |||||
PHAROS: P34897 GTEx: ENSG00000182199 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P34897 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TRS Query on TRS | I [auth A] | 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL C4 H12 N O3 LENZDBCJOHFCAS-UHFFFAOYSA-O |  | ||
| PEG Query on PEG | AA [auth D], J [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | E [auth A] F [auth A] G [auth A] H [auth A] L [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| NA Query on NA | BA [auth D], K [auth A], Q [auth B], W [auth C] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| LLP Query on LLP | A, B, C, D | L-PEPTIDE LINKING | C14 H22 N3 O7 P |  | LYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 59.184 | α = 90 |
| b = 125.571 | β = 93.675 |
| c = 135.137 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Polish National Science Centre | Poland | 2023/50/E/NZ7/00501 |